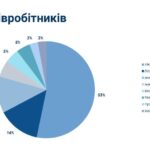
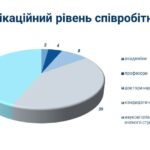
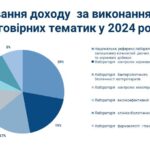
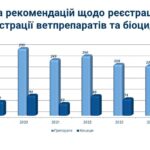
On July 2, a regular meeting of the State Pharmacological Commission on Veterinary Medicine of Ukraine was held at the State Service of Ukraine on Food Safety and Consumer Protection.
This stage is the final point in a complex and multi-level process of scientific evaluation of veterinary drugs and biocidal products.
Before any veterinary medicinal product or biocide is allowed onto the national market, it undergoes a comprehensive scientific assessment conducted by experts of the
State Scientific and Research Control Institute of Veterinary Medicinal Products and Feed Additives.
As part of the scientific evaluation:
clinical studies are analyzed separately (to assess efficacy);
toxicological evaluation is carried out (to assess safety);
the compliance of residue levels of active substances and the declared withdrawal periods with Ukrainian and EU legislation is assessed;
quality control of manufacturing processes is verified;
the impact of the product on human health, animal welfare, and the environment is evaluated;
laboratory testing of samples is performed at the Institute’s facilities.
The Commission’s decisions are based on the conclusions of an interdisciplinary scientific review, which ensures the quality, efficacy, and safety of veterinary medicines introduced to the Ukrainian market.
We sincerely thank the specialists of the State Service of Ukraine on Food Safety and Consumer Protection for their continued support, constructive dialogue, and contribution to this critically important work.
It is thanks to our close cooperation that we are collectively strengthening the country’s system of veterinary safety.